Our Research
Our Problem
Patients are affected by trauma induced heterotopic ossification from a variety of injuries and surgical procedures. HO is found in >65% of blast/burn injury, 20-80% of joint replacement, 20% of traumatic fractures and sports, and 20% of traumatic spinal cord injury patients.
This formation of bone in places where it is not normally found can lead to problems with:
- pain
- wound healing
- decreased joint range of motion
- drastic reduction in quality of life
- There is NO effective treatment or prevention of this disease in contemporary medicine. Furthermore, surgeons have their hands tied, as cutting the growth out with surgery can cause even more bone to form.
Strategies & Results
Our variety of models have and continue to facilitate our elucidation of key steps along a convoluted HO pathway. We have demonstrated that the formation of HO is due to dysregulation of inflammation and normal stem cells needed for proper repair going awry. Given many amazing scientists before us have carefully studied normal bone development, we are applying those lessons into clarifying the behind-the-scenes of HO formation to treat and prevent it from forming.
We’ve developed a mouse model that closely resembles the disease in humans, both post-traumatic and genetic varieties of HO (fibrodysplasia ossificans progressiva), to tackle the key questions behind the disease from many contexts. These investigations include the role of vascularity, mechanotransduction, and nerves just to name a few. To answer these questions, we rely on a myriad of state-of-the-art imaging techniques including micro CT, immunohistochemistry, and immunofluorescence.
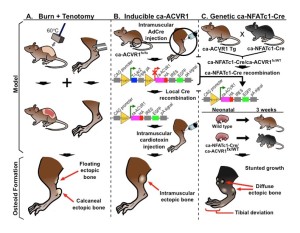
Burn + Tenotomy Model
Our fundamental trauma model consists of a concomitant dorsal burn and achilles tendon tenotomy. This model reliable produces HO at the local tenotomy site and nearby environments.
Inducible Geneotype
Many of our experiments rely heavily on inducible gene technology. Using Cre recombinase and lox-P site technology, we can create robust models whose genes of interest can be turned on and off at will.
Genetic Recombination
Stemming from our inducible gene schema, combined permutations expand our scope and types of interrogations we can apply to our HO pathway.
Collaborative Spaces
Second Harmonic Generation
3D in vivo
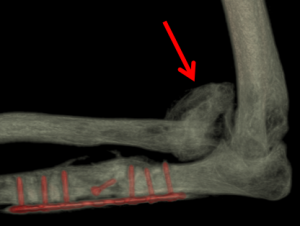
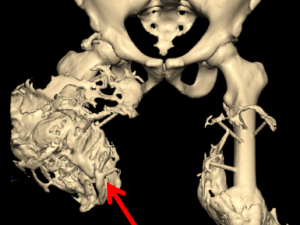
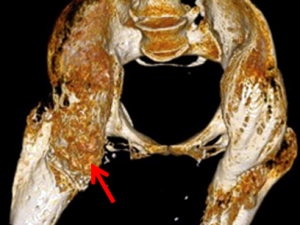
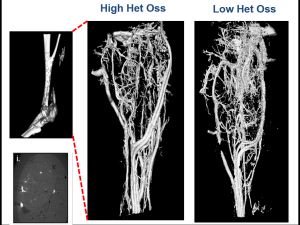
Micro CT Reconstructions
Micro CT reconstruction is a foundational component of much of our research allowing us visualization for exploration of our models and quantifying HO growth.
Heterotopic Ossification
Early diagnosis and Prevention strategies
As many as 65% of our severely combat-injured service members will go on to develop heterotopic ossification (HO), a musculoskeletal disorder characterized by the formation of mature bone in soft tissues including muscle, tendon, ligaments and fascia. As a complication of trauma, HO presents the most important barrier to functional recovery and independence.Furthermore, over 60% of civilian major burn patients and over 50% of joint replacement surgery patients develop HO, with risks that only increase after subsequent operations. The pervasive nature of HO extending across several tissue types suggests that treatment directed to a specific tissue may be ineffective, whereas therapy directed towards centrally acting pathways may be more effective. Once bone forms around a joint, patients develop debilitating joint contractures and loss of mobility. Surgical excision of HO can be attempted to restore function, but patients with periarticular HO rarely regain complete range of motion, with contractures due to persistent or recurring HO. Current medical strategies to prevent de novo or recurrent HO including glucocorticoids, bisphosphonates, and non-steroidal anti-inflammatory medications either have significant side effects and/or uncertain impact, possibly because they fail to target key pathways involved in HO formation. In addition to effective therapies, methods of identifying potential HO patients prior to radiographic diagnosis are needed to target early intervention to a population that is likely to benefit. Given the burden of HO disease in these populations, its high morbidity and suboptimal treatments, there is substantial need for early diagnosis and therapy to inhibit HO by targeting its causative processes.
Fibrodysplasia Ossificans Progressiva
One of the Earliest Clues
Fibrodysplasia ossificans progressive (FOP) is a rare autosomal dominant disorder of skeletal malformations and progressive extraskeletal ossification, a congenital disorder that mirrors our topic of focus. Subsequent studies of this parallel disease have elucidated biochemical pathway steps and agents such as the ALK2 receptor. ALK2 mutations have now been well described to play a role in patients with (FOP), and recent studies have implicated ALK2 as playing a more central role in cartilage condensation that can lead to subsequent bone formation in unwanted areas. Establishing more links of this nature is a powerful strategy in localizing relevant agents.
Levi Lab
| Equipment List |
| fluorescent and confocal microscopy |
| MicroCT scanners |
| IVIS imaging devices |
| 10X RNA sequencing facilities |
| a mouse genomics core. |
| Leica confocal microscope and Bruker |
| SKYSCAN 1272 CMOS EDITION – High-resolution 3D X-ray Microscopy |
| Cytation 5- digital microscope and microplate reader |
| 4D-Nuclefector System |
| PARAMETER Cabinet X-ray System |
|
Injury Models |
|
Burn |
|
Tenotomy |
|
Muscle Crush |
|
Volumentric Muscle Loss |
|
Fracture |
|
Calvarial Defect |
|
Digit Tip Regeneration |
Advanced Imaging Research Center for Mouse Imaging:
An IVIS Imaging System 200 Series (Caliper Life Sciences) for bioluminescence imaging
The Mouse Core Facility, run and maintain by the Advanced Imaging Research Center has Micro CT and small animal SPECT In vivo fluorescence and BLI (Xenogen IVIS Spectrum and Lumina units) and Ultrasound machine (Vevo 770, Visual Sonics). The CRI and Center for Mineral Metabolism both have MicroCT imagers.
Molecular Pathology Core
The Molecular Pathology Core maintains infrastructure and expertise for (i) embedding tissue samples; (ii) preparing plastic and paraffin sections, including hard-tissue processing and resin thinning (Buehler IsoCut Slow Speed Saw, PetroThin Grinder, and AutoMet 300/Ecomet 250 Polisher, Leica RM2255 Rotary Microtome outfitted for Tungsten-Carbide resin microtomy).
Bioinformatics
The hardware includes a PowerWulf Compute Engine, which contains 208 total processor cores, 800 GB system RAM, 84 TB Raw RAID6 storage with 10 GigE access to switch, and an Overland Tape Backup System, a Windows server for software, a database server, and a web server.
Confocal Microscopes: The Levi Laboratory has a live-cell compatible Leica Stellaris DM8 Confocal microscope with Leica LASX+ (NL8.122A) capable of super resolution and fluorescence lifetime analytics. The lab also maintains access to multiple Zeiss LSM 880 confocal/multiphotons (NL7.134A, K1.224 with Airyscan), an Andor spinning disk confocal with FRAP and TIRF (NL5.120R), and a Perkin Elmer Ultraview spinning disk confocal – BSL2 (Y9.326). Zeiss LSM 780 confocal/multiphoton (NL5.120N) Zeiss LSM 780 confocal/multiphoton (NL11.125A) Zeiss LSM 880 confocal/multiphoton (NL7.134A). Zeiss LSM 880 Airyscan confocal microscope (K1.224).Andor spinning disk confocal with FRAP and TIRF (NL5.120R) Perkin Elmer Ultraview spinning disk confocal – BSL2 (Y9.326)
Confocal Microscopes
The Levi Laboratory has a live-cell compatible Leica Stellaris DM8 Confocal microscope with Leica LASX+ (NL8.122A) capable of super resolution and fluorescence lifetime analytics. The lab also maintains access to multiple Zeiss LSM 880 confocal/multiphotons (NL7.134A, K1.224 with Airyscan), an Andor spinning disk confocal with FRAP and TIRF (NL5.120R), and a Perkin Elmer Ultraview spinning disk confocal – BSL2 (Y9.326). Zeiss LSM 780 confocal/multiphoton (NL5.120N) Zeiss LSM 780 confocal/multiphoton (NL11.125A) Zeiss LSM 880 confocal/multiphoton (NL7.134A). Zeiss LSM 880 Airyscan confocal microscope (K1.224).Andor spinning disk confocal with FRAP and TIRF (NL5.120R) Perkin Elmer Ultraview spinning disk confocal – BSL2 (Y9.326)
The Levi Lab Team
The BWR Laboratory is grounded on a foundation of teamwork, bringing together doctors, professional scientists, medical students, graduate students, and undergraduates from a variety of backgrounds.
Support Our Research
Burn/trauma and regenerative medicine researchers in plastic surgery seek to prevent the formation of bones in abnormal locations. Gifts help alleviate this condition affecting burn, auto accident, orthopaedic surgery and blast injury patients by providing additional resources to employ state of the art technologies, recruit talented researchers, and disseminate our scientific findings widely.